

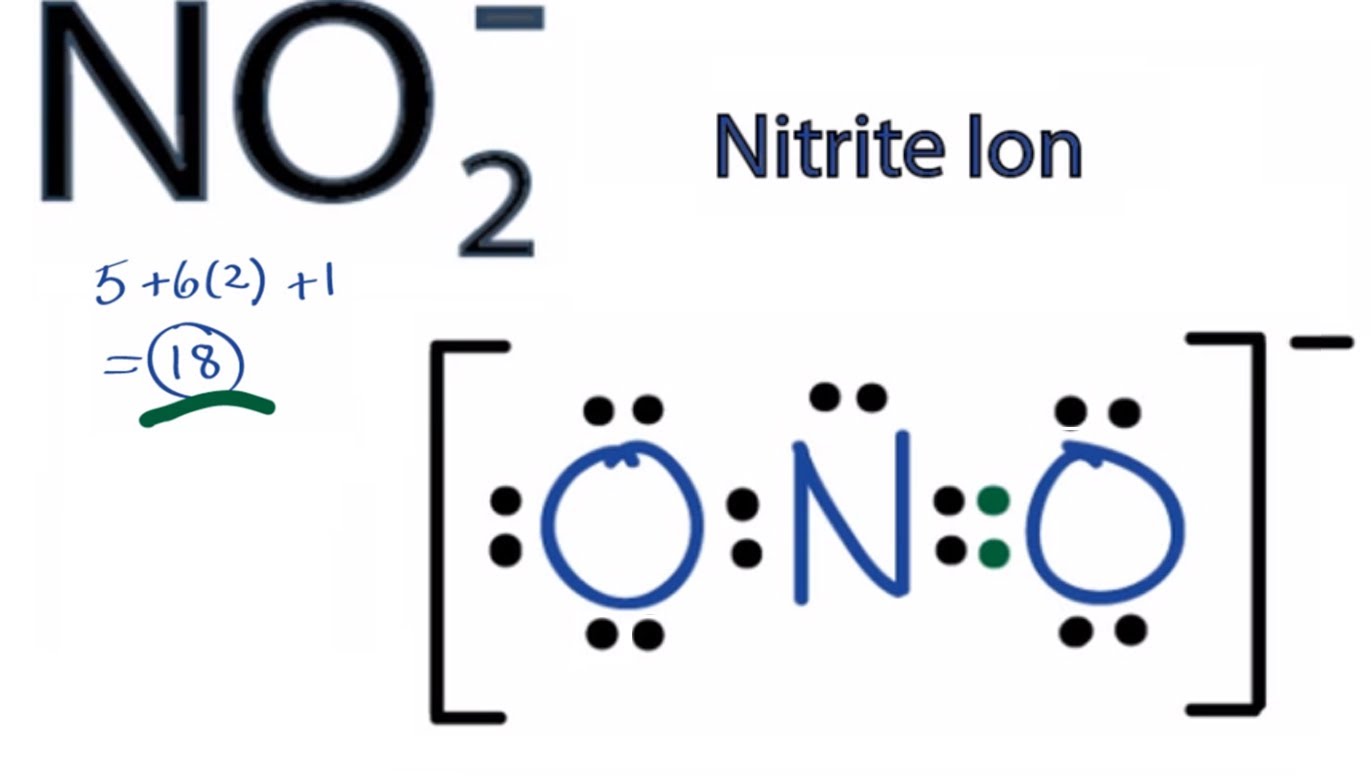
Oxygen has 6 valence electrons, and this atom has 2 bonded electrons and 6 unbonded, thus the FC is (6) – (½)(2) – (6) = -1. This one has 8 bonded electrons and no unbonded, thus the FC is (5) – (½)(8) – (0) = +1.įinally, we calculate the formal charge of the oxygen. Next, we calculate it for the nitrogen in the middle. Nitrogen has 5 valence electrons, this atom has 6 bonded electrons (a triple bond), and 2 unbonded electrons, thus the formal charge is (5) – (½)(6) – (2) = 0. Let’s figure out which structure is correct.įirst, we calculate the formal charge of the nitrogen on the left. If there are multiple structures that satisfy requirements 1-3, then the structure with negative formal charges on the more electronegative atoms is preferred.Įxample: shown below are three possible structures for N 2O.Adjacent atoms in a molecule should have opposite signs if charges are present.If there is no possible structure where all formal charges are zero, then the preferred structure is one with the least number of nonzero charges.The preferred molecular structure is one where all formal charges are zero, as opposed to one where some this value is not zero.There are even specific guidelines to help you figure this out: This clues us into the structure of a molecule if there are multiple options: the one with the least/lowest formal charges is the preferred structure. Ideally, an atom in a molecule wants to have a formal charge of zero: this is the lowest energy, and thus the most stable state for it to be in. We will explore some examples of nonzero charge below.

Note: though the formal charge in these two examples has been zero, that will not always be the case.
#Calculating formal charge. how to#
But more on that later! How to Calculate Formal Charge:įormal Charge (FC) = (# of valence electrons) – (½)(number of bonded electrons) – (number of unbonded electrons) Examples:ĬH 3O: what is the formal charge on the carbon?Ĭarbon has 4 valence electrons, 8 bonded electrons (two single bonds and one double bond), and no unbonded electrons. This is a hypothetical measure, not a real representation of the actual charge on an atom, which looks at the ways electrons are actually shared between atoms in a bond.
Quantifying protons, neutrons, and electrons.In this tutorial, you will learn what is formal charge, how to calculate it, and its significance in practice. Formal charge is an essential, basic concept to master in order to better understand molecular structures and reactions.


 0 kommentar(er)
0 kommentar(er)
